Colouring anodized aluminium
Explore the methods of colouring anodized aluminium and learn about the advantages of electrolytic colouring for achieving color uniformity and cost-effectiveness.
Colouring anodized auminium
Colour anodized aluminium was developed almost together to anodizing itself. The most important methods of colouring anodized aluminium are (Figure 1):
- Dying
- Integral colouring (self colouring)
- Electrilitic colouring (electrocolouring)
- Interference colouring.
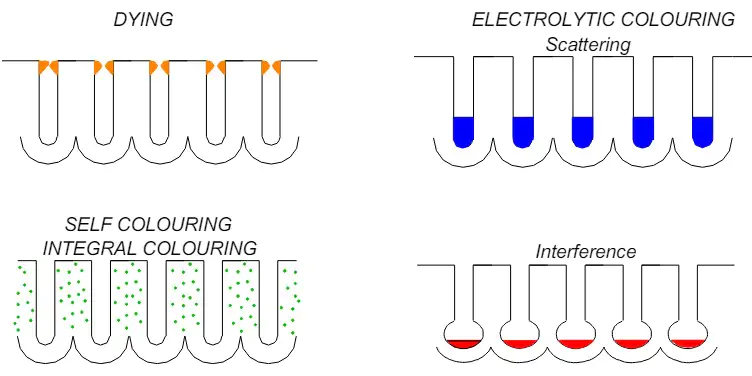

Figure 1 The methods of colouring anodising aluminium [1]
Electrolytic colouring anodized aluminium
The electrolytic coloring process consist of:
- Immersing the anodized work in a solution containing metallic salts
- Applying an AC or DC voltage.
- Under these conditions, metallic deposits are formed at the bottom of the pores.
- The colour depends on the nature of electrolyte.
- Metals tin, nickel and cobalt give a bronze to black colour.
- Copper gives red colours.
Main characteristics, by which this method has advantages:
- color uniformity;
- possibility of coloring thin anodic films;
- coloring speed;
- cost;
- light resistance;
- corrosion resistance.
Tin sulfate colouring
The most widely used electrolytes are tin sulfate ones. These electrolytes are given all the shades of “bronze”: from light champagne to light bronze and further to black bronze (Figure 1). Moreover, with the help of modern DC converters, you can get, for example, the special colors, such as “stainless steel”.
Influence of the original anodic films
The anodised film comprises:
- electrically conductive aluminium base;
- barrier oxide layer 50-2000 angstroms (2000 times thinner than the oxide layer) and
- a porous oxide layer thickness 10-20 micrometers.
Chemical composition
Electrocoloring is very sensitive to the chemical composition of an aluminium alloy, for example, to the content of such elements, as iron and silicon. Chemical composition of the aluminium alloy can affect the porosity of the anodic oxide layer, and, thereby, the tone of the resulting color.
Anodizing conditions
- Tin is deposited on the bottom of the various pore sizes.
- This leads to different conditions for the scattering of light and, consequently, different tones of color.
- The rate of deposition of tin ions within the pores depends on the dimensions of their cross-section.
- So, the uniformity of the porosity of the surface to be painted is a very important factor for the formation of a uniform color.
Barrier layer
- The uniformity of the barrier layer plays an important role in ensuring the formation of a uniform coating color.
- Since the barrier layer is an electrical insulator, it is an energy regulator for the electrocoloring.
- Therefore, differences in the thickness of the barrier layer result in differences in the deposition rate and, thereby, a non-uniform coloration.
The factors of electrolitic coloring anodised aluminium
Chemical factors:
- electrolyte type and concentration
- type additives and their concentration
- pH
- possible contamination
Physical factors:
- electrolyte temperature
- the electrical resistance of the electrolyte
- bath size and linkage with profiles
- the total surface area, which is painted
Electrical factors:
- the type and the supplied current waveform
- interval applied voltage
- material type cathodes
- schematic design cathode system in terms of electric current along the sample phase offset with profiles
- the distortion of the current wave shape as compared with the shape of the stress wave
- lines of magnetic induction electric fields
Deposition of tin in the anode pore
The basic facts:
- Electrocloring is a linear process from light tones to black (Figure 2).
- The dye, metallic tin, is located immediately above the barrier layer (Figure 3).
- A layer of tin in the pore grows perpendicularly pores base.
- Color anodizing coating arises due to the phenomenon of light scattering depends on the thickness and density of the deposited layer.
- For forming black required thickness of the deposited tin in the anode pore about 7-8 micrometers.
- The amount of metal tin in the pores: from 200 mg/m2 for light bronze to 2000 mg/m2 for black colour.

 Figure 2 – Kinetics of tin electrolitic deposition [2]
Figure 2 – Kinetics of tin electrolitic deposition [2]
Electricoloring speed
The electrocolouring speed (v) is a function of the concentration (C) of Sn2+ tin in solution:
v = KC
- An increase in the concentration (C) of Sn2+ tin makes it possible to reduce the duration of staining in one or another color.
- An increase in the coloring speed can also be achieved by increasing the reaction rate (K) instead of increasing the concentration of Sn2+ tin [2].
- Tin reduction reaction depends on the rate of reduction of H+. With an increase in the concentration of hydrogen ions (i.e., pH) the rate of tin reduction is increased.
- At the same time, the increase in the acidity of the bath has a limit. If the concentration of sulfuric acid exceeds 30 g / l, it may cause a defect in the form of delamination of the anode coating.
From divalent tin to tetravalent tin
- Typically, in the electrolyte on the basis of specific stabilizing additives tin sulfate are present in order to prevent spontaneous oxidation by dissolved oxygen of divalent tin ions Sn+2 tin ions to tetravalent Sn+4.
- Tetravalent tin ions Sn+4 undergo irreversible hydrolysis, which is accompanied by precipitation of sludge.
- The result is a spontaneous and irreversible loss of useful content stannous Sn+2.
- Therefore, the concentration of stabilizing additives is important. They slow down the reduction of the divalent tin concentration below the limit value. Below this limit value, the rate of deposition of tin in the pores of the anode layer is significantly slowing.
Sources:
- Anodizing of Aluminium – TALAT Lecture 5203 / José L.Gazapo and J. Gea, INESPAL Laminación, Alicante
- A New Economic Two-Step Coloring Procecc For Bronze And “Stainless Steel” Colors / W. D. Barba and F. Vincenzi – Aluminum ET Seminar, Chicago, 2004.

 Электролитическое окрашивание анодированного алюминия
Электролитическое окрашивание анодированного алюминия- Wrought construction aluminium: Eurocode 9
